Wellness

May 1, 2025
Don’t let Allergies Cloud your Spring
CRMC Pediatrician Wendi Johnson, MD, FAAP, recommends taking a proactive approach to allergy season to stay ahead of those sniffles and sneezes.
April 2, 2025
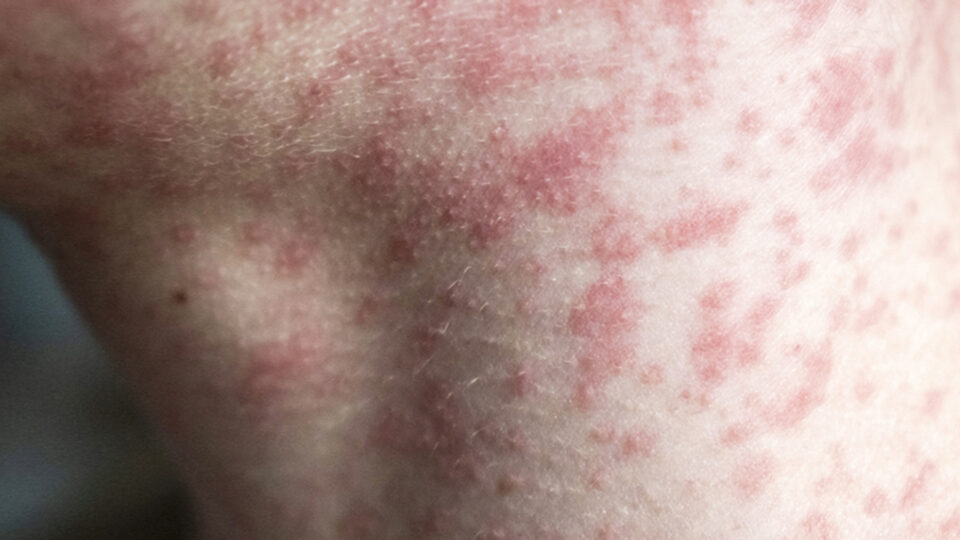
Stay Informed, Stay Protected: What You Need to Know About Measles
As Measles cases continue to pop up across the nation, you may be left feeling like you have more questions than answers. CRMC Pediatrician Wendi Johnson, MD, FAAP, shares some of the must-know facts surrounding the recent Measles outbreak.

February 23, 2025
Cancer Prevention: Human Papillomavirus (HPV) and why Vaccination Matters
HPV is a common virus that can lead to serious health complications in both men and women causing cervical and throat cancer, as well as other cancers. HPV is responsible for 90% of anal and cervical cancers and 70% of oropharyngeal cancers.

February 3, 2025
Don’t Let Seasonal Depression Go Unnoticed

October 30, 2024
Is it the cold, flu, or RSV?
Cold and flu season often packs a punch with symptoms like fever, sore throat, and congestion. No one wants to feel under the weather, especially with holiday gatherings and seasonal activities keeping schedules jam-packed. So, if you’re feeling crummy, what’s to blame? Is it the common cold, or could it be something more?

Coping with Holiday Stress
The hustle and bustle of the holiday season, often filled with joy and anticipation, can quickly turn dismal and dull. Before you know it, you’re left frazzled. Behavioral Health Psychologist Kristin Furan, PsyD, has some tips to help you cope with holiday stress.

August 7, 2024
Farmers Market Finds: The Benefits of Eating the Rainbow
“The deeper in color, or vibrant, the more nutritional complexity and benefits. Think of it like iceberg lettuce versus kale; the darker, deeper color of kale provides more benefits,” said Christenson.

Facing Cancer Together: Comprehensive Cancer Care and Support at CRMC
As an accredited cancer center through the Commission on Cancer, CRMC follows a multidisciplinary approach to treatment involving surgeons, medical and radiation oncologists, diagnostic radiologists, pathologists, and other specialists. The collaboration ensures that patients receive the best possible care.
Top Tips from a Physician for a Smooth Back-to-School Transition
Board-Certified Family Medicine Physician Hannah Elsenpeter, MD, has some advice for parents to help ease the transition from a carefree summer of fun into a routine that will have their kids ready to hit the books instead of snooze on their alarm.

July 29, 2024
Tick Alert: Safeguarding your Summer Activities
Mosquitoes and ticks can quickly spoil summer fun. If you enjoy spending time in nature or have pets that go outdoors, you need to be aware of these pesky parasites and the dangers they can potentially pose.

